Refining covalent warhead reactivity: A new look at GSH reactivity assays

If the bond is forever, it needs to be safe.
Covalent drugs have proved to be successful therapies for various indications. However, due to safety concerns, covalent compounds are not always considered when initiating a target-directed drug discovery project. Methods that can accurately assess the reactivity of irreversible and reversible covalent warheads at the start of a project would be valuable in derisking downstream drug development. We recently published a perspective in ACS Chem Biol on current approaches in covalent DEL technology and future steps to advance the field.
Current industry standard to measure warhead reactivity
In a drug discovery project, to ensure that potent covalent inhibitors are not promiscuous, electrophilic compounds are tested for reactivity with thiols. This measurement is carried out using a glutathione (GSH) reactivity assay, where a compound is incubated with excess GSH, and the consumption of the compound is measured by liquid chromatography-mass spectrometry to determine a pseudo-first order rate constant.1 A GSH half-life can then be determined from this rate constant. Safer drugs typically have a longer GSH half-life.
X-Chem’s new GSH assay solves limitations of industry standard GSH assay
The current industry standard GSH reactivity assay has several limitations. It treats irreversible and reversible covalent warheads in the same way. It is concentration-dependent, it is difficult to reproduce the same results across different labs and it is not always predictive of toxicity. At X-Chem, we developed a new GSH assay to address some of these limitations (Fig. 1).
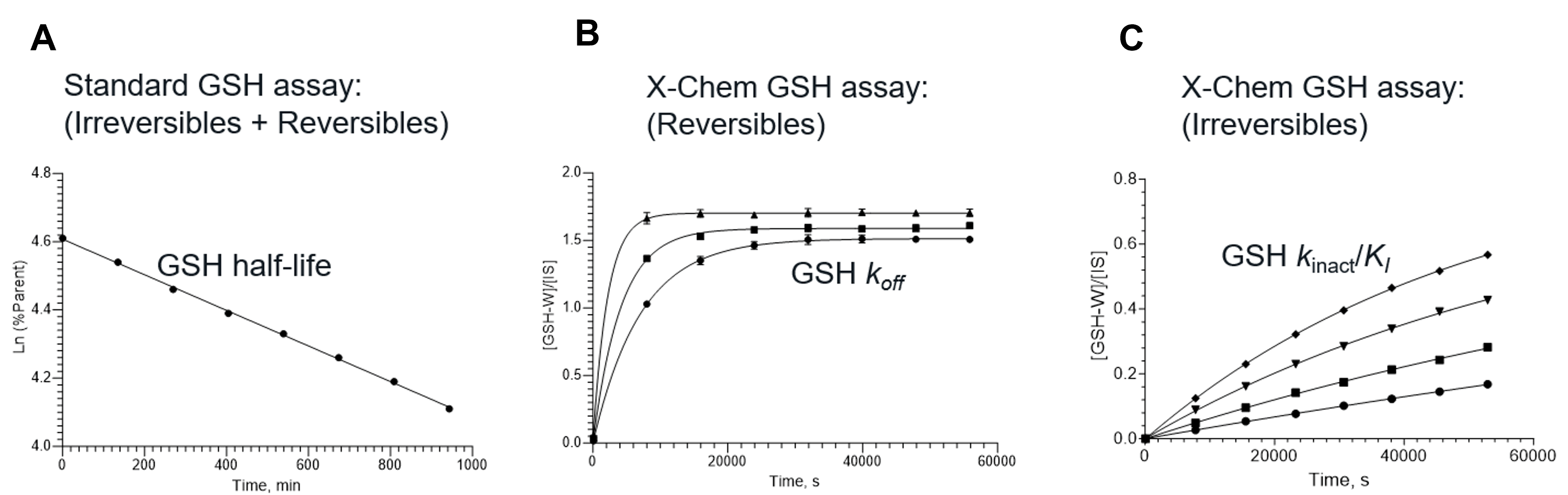
Our new GSH assay is like the industry standard in terms of the procedures involved in wet work, and is also high throughput, but we take additional parameters into consideration when analyzing the data, so that we can solve for the kinetics of warhead reactivity.
For example, for reversible covalent warheads, we use off-rate and not reactivity rate as a predictor for toxicity. We measure the equilibrium concentration of GSH-adduct at different GSH concentrations, then we determine Kd and off-rate (koff) for the reversible reaction. We assess toxicity by the difference between residence time (1/koff) on the protein target and on GSH.
For irreversible covalent warheads, we measure the increase of GSH-adduct concentration with time at different GSH concentrations. We then plot the different observed rate constants together to solve for kinact and KI. This method of assessing reactivity is akin to the FDA’s requirement for CYP inhibition and is also the method that covalent drug researchers are already using to assess on-target reactivity. We assess toxicity by the difference between kinact/KI for the protein target and GSH.
Winning at the beginning: Optimized covalent building blocks guarantee greater success
By changing how we extract kinetic constants from reactivity data, we can generate predictors for toxicity and differentiate the kinetics for reversible and irreversible warheads. Moreover, our readouts are in line with the best practice for adduct analyses of covalent proteins. This assay not only helps us design and incorporate more refined covalent building blocks (the structures of which are based on approved and late-stage drugs that target Cys, Lys, Ser) for our DNA-encoded libraries for screening, but also provides our partners with better tools to optimize and derisk their covalent hits and leads.
Reference
- Boike, L. et al. Advances in covalent drug discovery. Nat Rev Drug Discov., 2022, 21, 881–898.
Additional Reading:
- Gai, C. et al. Advanced approaches of developing targeted covalent drugs. RSC medicinal chemistry, 2022, 13(12), 1460-1475.
- Faridoon, Ng R. et al. An update on the discovery and development of reversible covalent inhibitors. Medicinal Chemistry Research, 2023, 32(6), 1039-1062.
- Strelow, J.M. A Perspective on the Kinetics of Covalent and Irreversible Inhibition. SLAS Discovery, 2017, 22(1), 3–20.
- Brum, L. In Vitro Drug Interaction Studies – Cytochrome P450 Enzyme and Transporter-Mediated Drug Interactions Guidance for Industry.
The Importance of Selecting the Right CRO Partners for Drug Discovery Success
When it comes to drug discovery and development, having the right team and partners is critical for success. For a...
AI-Powered Building Blocks “ReadiBLOX” for DEL Synthesis
Drug Hunter recently wrote the article “Decoding DNA-Encoded Libraries for Drug Discovery.” Over the past two decades, DEL technology has...